Explanation Of Ethylenediamine tetraacetate:
by : Rahul Sharma
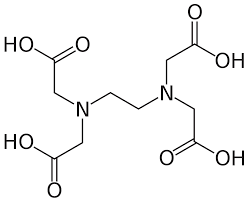
Ethylenediamine tetraacetate:
Ethylenediamine tetraacetate (EDTA) can form a few different water soluble salts with calcium, potassium and sodium, for example, calcium disodium, trisodium and tetrasodium salts.It is an aminopolycarboxylic acid and a colourless, water-soluble solid. Its conjugate base is ethylenediaminetetraacetate. It is widely used to dissolve limescale. EDTA tetrasodium salt is used most widely in many industrial applications as a powerful chelating agent. Its 1% solution has a pH of 11.3. It can chelate with many divalent and trivalent metal ions to form water soluble metal complexes.
