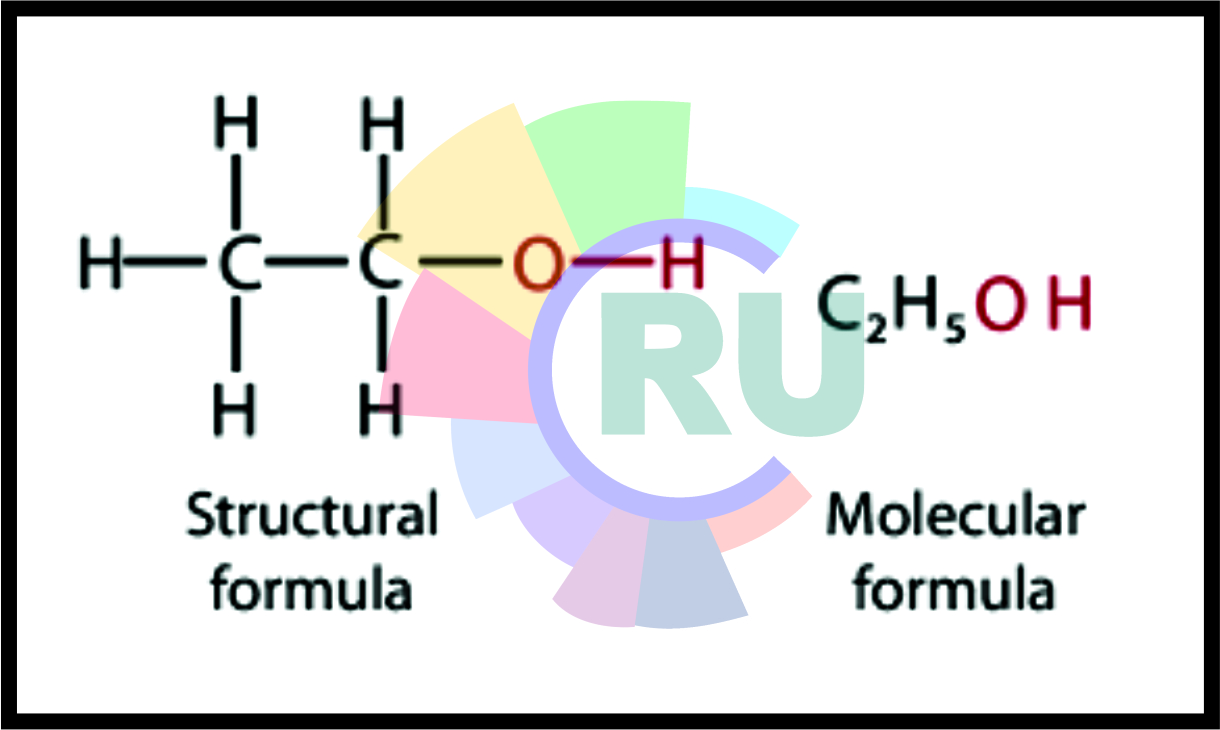
Ethanol CH3-CH2-O-H:-
by : ---
Ethanol CH3-CH2-O-H:-
Chain alcohols have their simple bonds derived from corresponding alkanes. For this reason, we call this type of alcohol aliphatic. These alcohols form a homologous series whose members have the general formula CnH2n+1OH. The name of these alcohols comes from the corresponding alkane plus the suffix -ol. Just as the alkanes, isomeres exist in this alcohol series, beginning with propanol. The placement of the hydroxyl group is indicated by a numbering system which pinpoints the carbon to which the group is bonded, with that number placed either before or after the name of the molecule.
The oxygen atom strongly attracts the electron pair it shares with carbon. Combined with two other electron pairs already found on the oxygen atom, the oxygen represents a partial negative charge compared to the rest of the molecule. The hydroxylic group is hydrophilic, meaning that it attracts water. The rest of the molecule, the hydrocarbon chain, is hydrophobic, meaning that it repels water.
